Heating Hydrochloric Acid Safely and Effectively
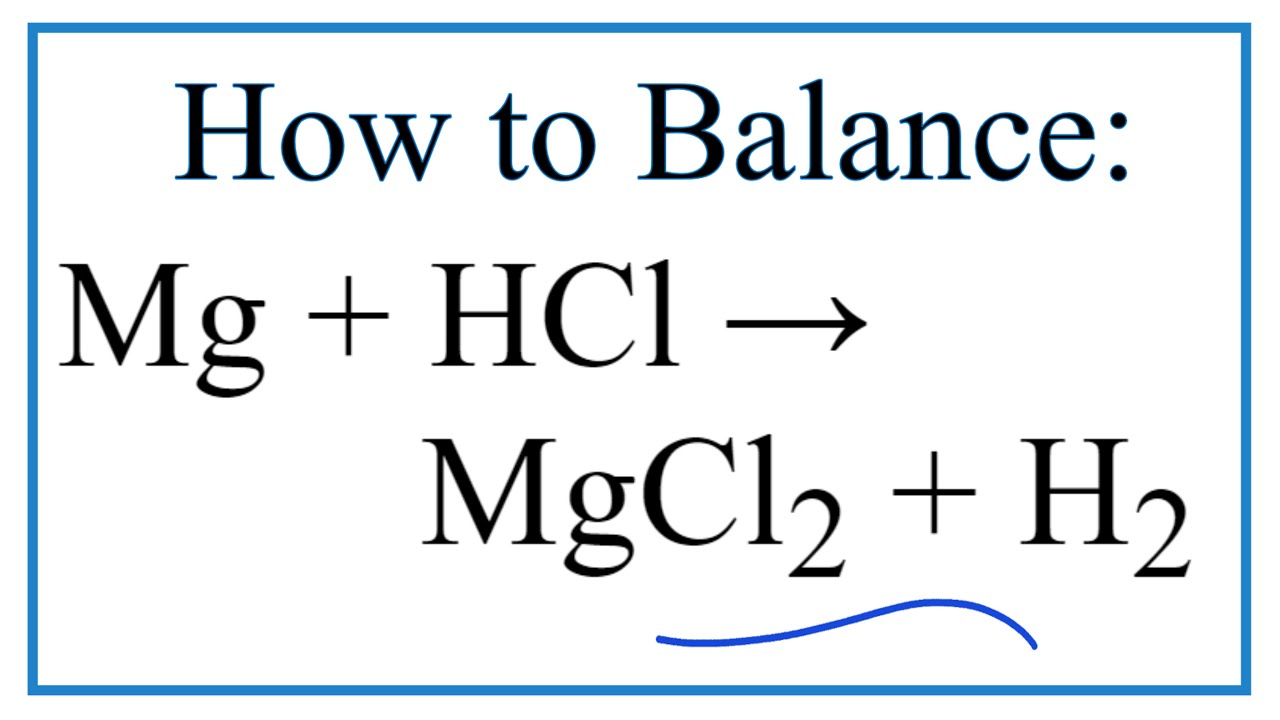
The subtle dance between chemistry and practicality often leads us to explore the manipulation of substances, sometimes through seemingly simple actions like applying heat. When it comes to a potent chemical like hydrochloric acid, understanding the implications of raising its temperature becomes crucial. This exploration delves into the nuances of heating hydrochloric acid, addressing the safety concerns, best practices, and potential applications involved in this process.
Hydrochloric acid, a strong mineral acid, plays a vital role in various industrial, laboratory, and even household settings. Its reactivity makes it an invaluable tool for cleaning, etching, and pH adjustment. But with its power comes a responsibility to handle it with care, especially when introducing heat into the equation. Applying heat to hydrochloric acid can intensify its corrosive nature, leading to the accelerated release of harmful fumes and increasing the risks associated with its handling.
The history of hydrochloric acid is intertwined with the alchemists of the Middle Ages, though its pure form wasn't isolated until the 17th century by Johann Rudolf Glauber. Its importance has only grown with time, becoming an essential component in modern industrial processes. From metal refining to the production of PVC, hydrochloric acid's versatility has cemented its place as a key chemical reagent.
One of the main issues related to heating hydrochloric acid is the release of hydrogen chloride gas. This pungent, corrosive gas poses a significant risk to respiratory health and can cause severe burns to the eyes and skin. Therefore, any procedure involving the application of heat to hydrochloric acid necessitates meticulous safety precautions and controlled environments.
Applying heat to hydrochloric acid is not a trivial matter. The increased temperature can significantly impact reaction rates, influencing both the speed and outcome of chemical processes. In certain applications, gentle heating can facilitate desired reactions, while excessive heating can lead to undesirable consequences, including the decomposition of the acid itself.
Several benefits can arise from carefully controlled heating of hydrochloric acid solutions. For example, in some chemical syntheses, heating the acid can drive a reaction towards completion more quickly. In specific cleaning applications, a warm hydrochloric acid solution can be more effective at dissolving stubborn stains or mineral deposits. Also, in certain industrial settings, heating hydrochloric acid can aid in the purification of specific materials.
A detailed action plan for safely heating HCl involves meticulous preparation. This includes ensuring adequate ventilation, using appropriate personal protective equipment (PPE) such as gloves and eye protection, and conducting the process within a controlled laboratory environment. Slow and controlled heating is paramount to prevent rapid gas evolution and potential splashing.
Advantages and Disadvantages of Heating Hydrochloric Acid
| Advantages | Disadvantages |
|---|---|
| Increased reaction rates | Increased risk of HCl gas release |
| Enhanced cleaning effectiveness | Elevated corrosion potential |
| Facilitates specific purification processes | Risk of thermal decomposition |
Best Practices:
1. Always work in a well-ventilated area.
2. Wear appropriate PPE, including gloves, eye protection, and a lab coat.
3. Use a hot plate with a magnetic stirrer for controlled and even heating.
4. Never heat hydrochloric acid in a closed container.
5. Add acid to water slowly, never the reverse.
Frequently Asked Questions:
1. Can I heat hydrochloric acid on a stovetop? No, it’s unsafe to heat hydrochloric acid on a stovetop due to the risk of uncontrolled heating and fume release.
2. What type of container should I use for heating hydrochloric acid? Use heat-resistant borosilicate glassware specifically designed for laboratory use.
3. What precautions should I take when heating hydrochloric acid? Always wear appropriate PPE and work in a well-ventilated area. Use a hot plate with a magnetic stirrer for controlled heating.
4. What should I do if I inhale hydrochloric acid fumes? Immediately move to fresh air and seek medical attention if necessary.
5. Can I heat hydrochloric acid in a microwave? No, heating hydrochloric acid in a microwave is extremely dangerous due to the risk of uncontrolled heating and potential explosion.
6. How can I dispose of heated hydrochloric acid? Follow proper chemical waste disposal procedures as outlined by your local regulations.
7. What is the boiling point of hydrochloric acid? The boiling point varies depending on the concentration of the acid.
8. Is it safe to heat dilute hydrochloric acid? Even dilute hydrochloric acid can release harmful fumes when heated, so proper precautions are still necessary.
In conclusion, heating hydrochloric acid can be a valuable technique in certain applications, from accelerating chemical reactions to enhancing cleaning processes. However, the inherent hazards associated with this potent acid necessitate a meticulous approach. By adhering to strict safety protocols, employing appropriate equipment, and following best practices, the risks can be minimized, and the benefits of controlled heating can be effectively harnessed. Understanding the chemical properties of hydrochloric acid, respecting its potential dangers, and implementing careful procedures are essential for anyone working with this powerful substance. Safety should always be the paramount concern when dealing with hydrochloric acid and the application of heat.
Taming the tide a deep dive into boat washdown pumps
Decoding the phenomenon of lil tonys money focused music
Conquering the internet the reign of the cute cat pfp drawing













