Unveiling the Magic of Neutralization Reactions

Ever wondered how indigestion tablets work their magic? Or how farmers adjust soil pH for optimal crop growth? The answer lies in a fundamental chemical process called neutralization. It's a constant presence in our lives, from the mundane to the extraordinary, shaping our world in ways we might not even realize.
Neutralization chemistry, at its core, is the reaction between an acid and a base, resulting in the formation of a salt and water. This interaction essentially cancels out the acidic and basic properties, creating a more neutral solution. Think of it as a chemical balancing act, where the extremes of pH are brought closer to the middle ground.
This seemingly simple reaction underpins numerous natural phenomena and industrial processes. From the regulation of ocean acidity to the production of everyday household products, neutralization reactions play a crucial role. Understanding this process opens a window into a fascinating world of chemical interactions and their impact on our environment and daily lives.
The history of understanding neutralization traces back centuries, with early alchemists and chemists observing the interaction between acidic and alkaline substances. Over time, the development of the pH scale and a deeper understanding of acid-base chemistry provided a more precise framework for studying these reactions. Scientists like Svante Arrhenius and Johannes Brønsted contributed significantly to our modern understanding of how acids and bases behave in solution and how neutralization occurs.
The importance of neutralization reactions is undeniable. In environmental science, it helps buffer the impact of acid rain on ecosystems. In medicine, it's the principle behind antacids that neutralize excess stomach acid. In agriculture, farmers use neutralization techniques to adjust soil pH for optimal plant growth. Without neutralization, our world would be a drastically different and less hospitable place.
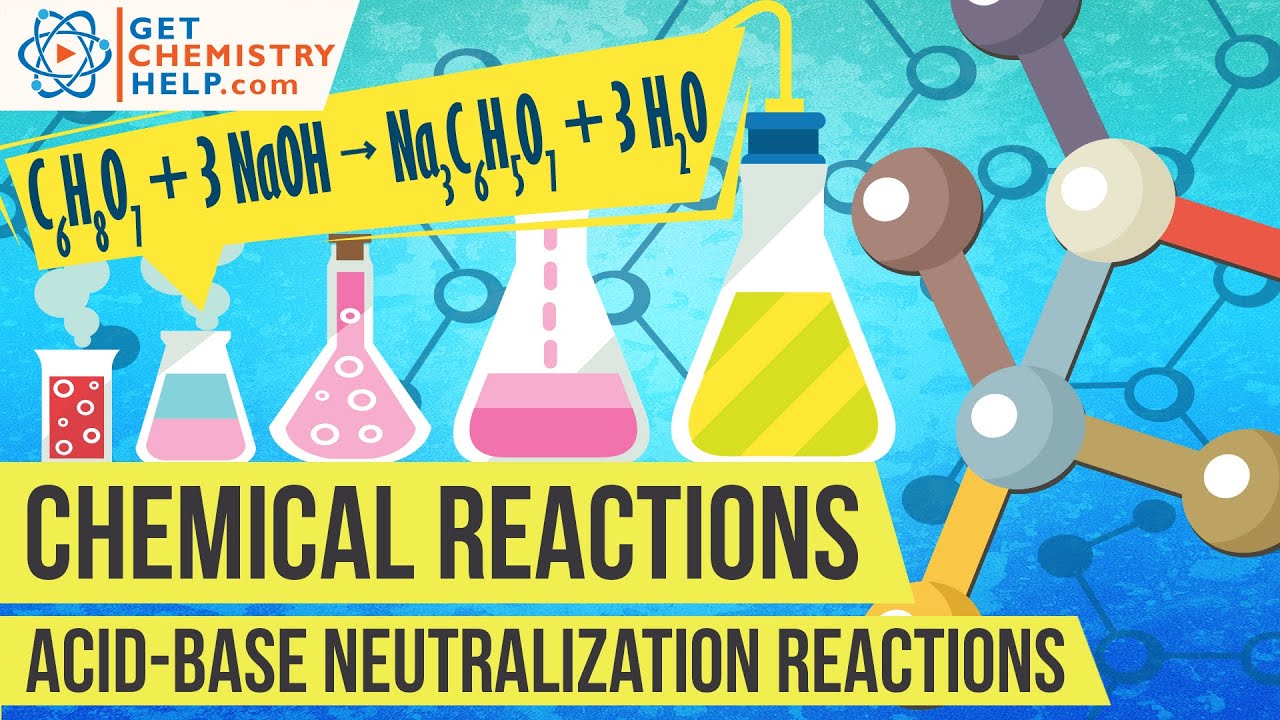
A simple example of a neutralization reaction is the combination of hydrochloric acid (HCl) and sodium hydroxide (NaOH), a strong base. The reaction produces sodium chloride (NaCl), common table salt, and water (H₂O): HCl + NaOH → NaCl + H₂O. This example showcases the fundamental principle of how an acid and a base react to form a neutral solution.
One benefit of neutralization is its ability to treat acidic wastewater. Industrial processes often generate acidic waste, which can be harmful to the environment. Neutralization with a base allows for safe disposal or reuse of the treated water.
Another benefit lies in its application in medicine. Antacids neutralize stomach acid, relieving heartburn and indigestion. This simple application of neutralization chemistry brings comfort to millions of people daily.
Finally, neutralization plays a critical role in agriculture. Soil pH greatly influences plant growth. Farmers often use lime (calcium oxide) to neutralize acidic soils, creating a more suitable environment for crops.
A simple action plan for demonstrating neutralization involves mixing vinegar (acetic acid) with baking soda (sodium bicarbonate). Observing the fizzing reaction, the release of carbon dioxide gas, indicates that neutralization is occurring. This simple experiment can be easily performed at home or in a classroom setting.
Advantages and Disadvantages of Utilizing Neutralization Reactions
| Advantages | Disadvantages |
|---|---|
| Wastewater treatment | Potential for over-neutralization |
| Medical applications (antacids) | Salt formation can sometimes be problematic |
| Soil pH adjustment | Requires careful monitoring of pH levels |
A best practice for implementing neutralization in industrial settings is continuous pH monitoring. This ensures the reaction proceeds efficiently and avoids over-neutralization, which can lead to unwanted alkaline conditions.
A real-world example of neutralization is the use of limestone to mitigate the effects of acid rain in lakes. The limestone reacts with the acidic rainwater, neutralizing the acidity and protecting aquatic life.
A common challenge in neutralization is determining the correct amount of base needed to neutralize a specific acid. Careful calculations and titrations are crucial to avoid over or under-neutralization.
Frequently Asked Questions: What is neutralization? How does it work? What are examples? What are the benefits? What are the challenges? How is it used in industry? How is it used in medicine? How can I perform a simple neutralization experiment?
A tip for working with neutralization reactions is to always add the base to the acid slowly and with stirring. This prevents a rapid reaction that could cause splashing or overheating.
Neutralization chemistry is a fundamental process that quietly shapes our world. From treating indigestion to protecting our environment, its importance is undeniable. Understanding the principles of neutralization empowers us to harness its benefits for a wide range of applications. By exploring the intricate dance between acids and bases, we gain a deeper appreciation for the chemical reactions that underpin so many aspects of our lives. Whether it's the simple act of taking an antacid or the complex processes involved in wastewater treatment, neutralization chemistry is a constant force at work, improving our lives and protecting our planet. Continue exploring the fascinating world of chemistry and discover how even the simplest reactions can have profound impacts.
Unlocking top round roast perfection mastering cook time
Navigating the jefferson county jail ny system
The enduring allure of grey kitchen cabinets sherwin williams








/200448568-001-56a12eb35f9b58b7d0bcd858.jpg)


